One of our clients recently told our team member Rachel Knight that she is the friendliest quality person she’d ever met. Rachel was advising them on achievable ways to meet a particular accreditation requirement.
She was, naturally, quite pleased to get that feedback.
But it got us thinking…what does this say about all the other quality people out there? Could there be a cohort of quality managers nagging their laboratory staff to get things done – taking the “Just Do It” approach to quality?
That may be true for some quality managers, but it was reassuring to note that the 2024 World Quality Day theme was ‘From Compliance to Performance’. This was the whole reason I hung out my shingle as a NATA quality consultant! As I read Kevin Sanders‘ article in the last issue of Quality Business [‘The Modern Auditor – Supporting Organisations in their Success Journeys’], I thought, “he sounds like me”.
Kevin’s article focussed on what external auditors could do to give more value in the certification process, helping organisations move from a compliance mentality to a growth and improvement approach. The scope for developing an improvement culture is even greater for in-house quality personnel and auditors who don’t need to worry too much about offering advice.
The scope for developing an improvement culture is even greater for in-house quality personnel and auditors who have more scope to offer advice.
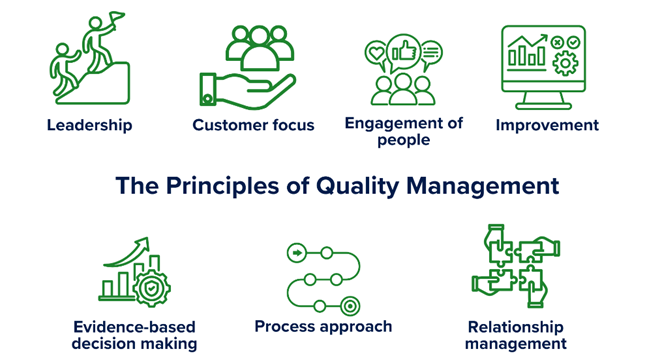
For those who don’t know, the standards for laboratory management are structured in line with ISO 9001 and follow the same guiding principles we all know and love:
Figure 1. The ISO Quality Principles
But, somehow in the laboratory accreditation system, these principles get lost in the minutiae of what I call ‘compliance thinking’. This is where we become so focused on compliance that we lose track of where the requirements come from and what their intention is, let alone focus on their guiding principles!
The evidence for this is clear when we see
- Staff recording all sorts of information on log sheets, saying “NATA said we have to do it” with no other reason for it.
- The data recorded is not used to inform decision-making.
- Awkward/extra steps slowing down processes or methods “because NATA said” or “because it’s a requirement of the standard”.
- The laboratory is reluctant to drop particular calibration or QC activities that they know are unnecessary because they don’t know how to justify their decision in the context of accreditation.
- The documented quality system doesn’t help people to do their job. It’s just there for show.
These are all signs of a compliance-focused quality system.
Many laboratory people have been misled about what the “quality management system” really is. They think it’s the quality manual or procedures covering document control, internal audits, management review, etc. That’s not it. Those are documents that describe the QMS.
Many laboratory people have been misled about what the “quality management system” really is. They think it’s the quality manual or procedures covering document control, internal audits, management review, etc. That’s not it. Those are documents that describe the QMS.
The real quality system is all the things you do to create reliable, traceable results and reports. It’s how you keep your customers happy.
Why does ‘compliance thinking’ happen?
This sort of unhelpful focus on documentation and records can happen when:
❌ You are worried or embarrassed about getting findings in NATA assessments, so you decide that the laboratory must do everything that every NATA assessor has ever suggested you do.
❌ NATA asks you to do specific things, but you don’t have time to work out what the true root cause was, so you’re not sure if you have addressed the real issue.
❌ You’ve taken the “NATA is the expert” approach and just accepted all their findings without questions. This is where your quality system devolves into a series of unrelated add-ons suggested by NATA auditors.
❌ You’ve copied your quality manual from a friend / the internet/ a “certification guaranteed” consultant.
How can you stay true to the principles and have NATA Accreditation?
This is where you get to develop a reputation as a “friendly quality person”. By keeping those principles of ‘customer focus’, ‘leadership’, ‘engagement of people’, ‘process approach’, ‘improvement’, ‘evidence-based decision making’, and ‘relationship management’ in mind, you will find that you develop a strong working relationship with your laboratory personnel, making it easier to keep improving.
Showing empathy with laboratory staff
Showing empathy doesn’t mean you “give in” or back away from requirements. It means you take the time to understand their situation. If you have to ask laboratory staff to do yet another task, make sure you have done all the background work first:
- Study the finding or requirement, gather examples and make sure you know how this applies in your laboratory and how it will control risk or improve your service.
- Study the current process with them to see if it’s working well and how it might already meet the requirement.
- Work with them to meet the requirement by building onto existing systems that are working well.
- When new processes are implemented, include training so everyone gets the facts behind the new process.
- Check in with your people from time to time. Revisit the findings and be prepared to accept feedback from the team about how it’s working.
Don’t leave staff grumbling about doing extra tasks without making sure they understand WHY they are doing them.
Involving staff in the changes
Do try to get the laboratory staff to come up with the details of how to meet the requirements. Work with them to ensure the system is efficient and effective but empower them to make changes themselves.
Make the quality system visible and easy to follow
Making a quality system visible entails setting up your workplace so that everyone knows what to do or can look it up easily. Having a logical workflow, with some signage and tape to define areas on benches may be all that’s needed for day-to-day operations to flow smoothly. Add automation into the mix and there is even less need for written procedures.
Any written procedures should help people to do their job and define processes that benefit from being repeatable (e.g. test methods). Quality manuals and procedures that are “just for NATA” create waste. By the time you’ve had a few assessments, you will have a weird chimera of generic policies and specific details as requested by NATA auditors. There will be duplication, confusion and more assessment findings because no one really understands the content.
Use evidence for decision-making
One of the things that can really frustrate staff is being told to “Just Do It”. While just doing it is great if you’ve decided you need to build a new habit, it’s not so great if this is how you respond to assessment findings.
Do the research, understand where assessment findings are coming from and do not hold back from asking NATA to clarify requirements (i.e. provide evidence to support their finding). You owe this to your staff.
At the other end of the spectrum, many labs gather data but don’t use it for anything. Let’s look at external calibrations as an example. You send gadget X out for calibration every year for 10 years or more. You pay the bill, check the results showed X is OK to go back into service and file the calibration report. At every NATA assessment they look at the calibration reports and record nothing. Because there’s nothing to see here, right?
If you have 10 years of data showing X is stable and always within acceptance limits without adjustment, how could you use that data? Could you save money and effort by extending the calibration period?
It’s even more of a crime if you’re asking laboratory staff to record data (e.g. who cleaned the biohazard cabinet and when) and you don’t make any decisions based on that data. You can likely think of many other examples of this kind!
And finally, you need evidence to decide whether improvements made have yielded any benefits. By reporting these benefits back to staff, they start to see how what they are doing (that may otherwise seem a little pointless) contributes to your improvement culture.
Involving leadership
Knowing when you need to include the senior management team in your NATA responses is critical. These are the people who are best placed to hold you back from the “Just Do It” urge and understand how your customers might view any changes made.
Make the Laboratory / Quality Principles your guide
It’s so easy to end up down in the weeds, discussing HOW to implement changes NATA has asked for after a NATA assessment. Instead, we should be doing a thorough investigation and cause analysis to confirm NATA’s findings before we jump into action. Involving the leadership team will make your staff feel more confident about the change.
Our seven quality principles are invaluable here. Ask yourselves the following key questions after you have investigated and got all the facts:
- Does this change improve our process or method? I.e. make it more reliable, accurate, or relevant? (Process approach)
- What is the evidence that the change is needed? (Evidence-based decision-making)
- How will this change impact our customers? Will they like it? Would they pay for it? Inform them before they are impacted! (Customer focus, relationship management)
- Will there be a measurable improvement if we do it? (Improvement)
- Has our management team approved the change and provided the necessary resources? (Leadership)
- What do our staff think about it? There might be benefits or risks we haven’t thought of. (Engagement of people, relationship management)
It’s your lab, your quality system; you get to decide which changes need to be made and how.
Remember, a quality management system isn’t the quality manual or procedures covering document control, internal audits, management review, etc. They are the documents that describe the QMS.
The quality system is everything you do to create reliable, traceable results and reports. It’s how you keep your customers happy. Focus on those processes when you’re looking for something to improve.
This article was published in Quality Business 2025 Vol 1 Jan – Mar . You’ll need to log in as a member to read it there.
If you’d like some tips on how to understand and reply to problematic assessment reports, grab a copy of my eBook.